The Element Nitrogen
N I Ground State 1s 2 2s 2 2p 3 4 S° 3 / 2 Ionization energy 117225.7 cm-1 (14.5341 eV) Ref. M75a N II Ground State 1s 2 2s 2 2p 2 3 P 0 Ionization energy 238750.3 cm-1 (29.6013 eV) Ref. Atomic number of an element never changes: for example, the atomic number of oxygen is always 8, and the atomic number of Chlorine is always 18. The atomic number is marked with the symbol Z, taken from a German word zahl (or atomzahl, which is 'atomic number' in German).
[Click for Isotope Data]
Atomic Number: 7
Atomic Weight: 14.00674
Melting Point: 63.15 K (-210.00°C or -346.00°F)
Boiling Point: 77.36 K (-195.79°C or -320.44°F)
Density: 0.0012506 grams per cubic centimeter
Phase at Room Temperature: Gas
Element Classification: Non-metal
Period Number: 2
Group Number: 15
Group Name: Pnictogen
What's in a name? From the Greek words nitron and genes, which together mean 'saltpetre forming.'
Say what? Nitrogen is pronounced as NYE-treh-gen.
Atomic Number 78

History and Uses:
Nitrogen was discovered by the Scottish physician Daniel Rutherford in 1772. It is the fifth most abundant element in the universe and makes up about 78% of the earth's atmosphere, which contains an estimated 4,000 trillion tons of the gas. Nitrogen is obtained from liquefied air through a process known as fractional distillation.
The largest use of nitrogen is for the production of ammonia (NH3). Large amounts of nitrogen are combined with hydrogen to produce ammonia in a method known as the Haber process. Large amounts of ammonia are then used to create fertilizers, explosives and, through a process known as the Ostwald process, nitric acid (HNO3).
Nitrogen gas is largely inert and is used as a protective shield in the semiconductor industry and during certain types of welding and soldering operations. Oil companies use high pressure nitrogen to help force crude oil to the surface. Liquid nitrogen is an inexpensive cryogenic liquid used for refrigeration, preservation of biological samples and for low temperature scientific experimentation. Jefferson Lab's Frostbite Theater features videos of many basic liquid nitrogen experiments, such as this one:
Estimated Crustal Abundance: 1.9×101 milligrams per kilogram
Estimated Oceanic Abundance: 5×10-1 milligrams per liter
Number of Stable Isotopes: 2 (View all isotope data)
Ionization Energy: 14.534 eV
Oxidation States: +5, +4, +3, +2, +1, -1, -2, -3
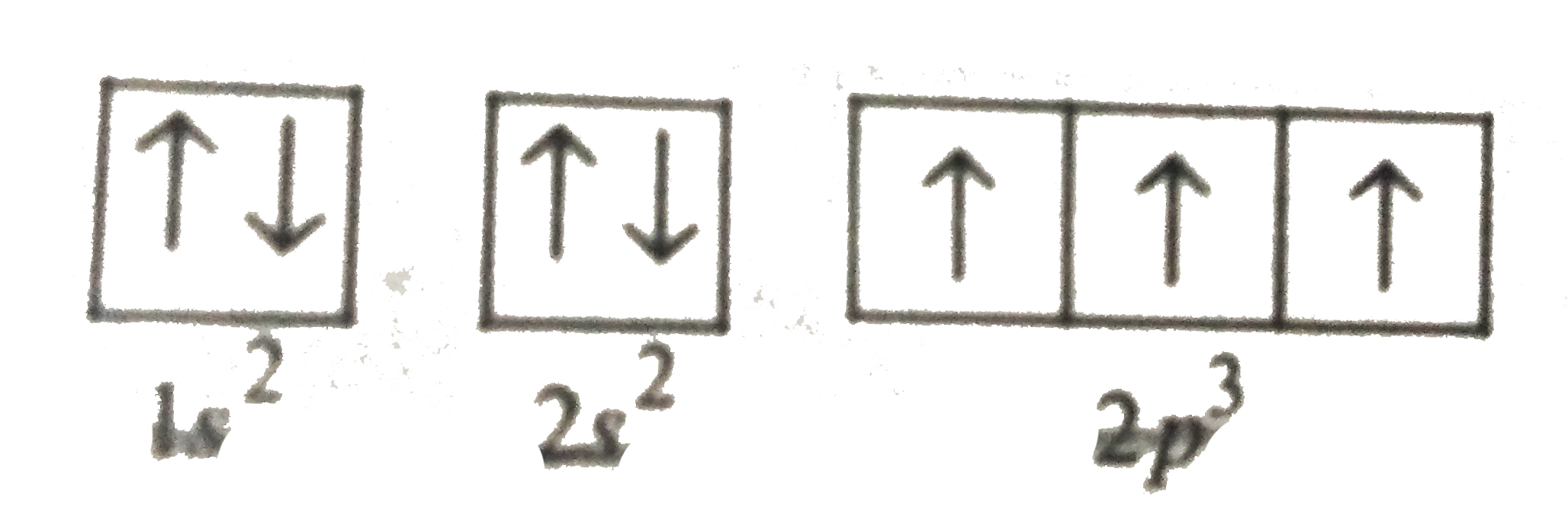
Electron Shell Configuration: | 1s2 |
2s2 2p3 |
For questions about this page, please contact Steve Gagnon.

The elements of the periodic table sorted by atomic number
click on any elements name for further chemical properties, environmental data or health effects.
This list contains the 118 elements of chemistry.
| The chemical elements of the periodic chart sorted by: | Atomic number | Name chemical element | Symbol |
| - Name alphabetically | 1 | Hydrogen | H |
| - Atomic number | 2 | Helium | He |
| - Symbol | 3 | Lithium | Li |
| - Atomic Mass | 4 | Beryllium | Be |
| - Electronegativity | 5 | Boron | B |
| - Density | 6 | Carbon | C |
| - Melting point | 7 | Nitrogen | N |
| - Boiling point | 8 | Oxygen | O |
| - Vanderwaals radius | 9 | Fluorine | F |
| - Year of discovery | 10 | Neon | Ne |
| - Inventor surname | 11 | Sodium | Na |
| - Elements in earthcrust | 12 | Magnesium | Mg |
| - Elements in human body | 13 | Aluminum | Al |
| - Covalenz radius | 14 | Silicon | Si |
| - Ionization energy | 15 | Phosphorus | P |
For chemistry students and teachers: The tabular chart on the right is arranged by Atomic number. The first chemical element is Hydrogen and the last is Ununoctium. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | 16 | Sulfur | S |
| 17 | Chlorine | Cl | |
| 18 | Argon | Ar | |
| 19 | Potassium | K | |
| 20 | Calcium | Ca | |
| 21 | Scandium | Sc | |
| 22 | Titanium | Ti | |
| 23 | Vanadium | V | |
| 24 | Chromium | Cr | |
| 25 | Manganese | Mn | |
| 26 | Iron | Fe | |
| 27 | Cobalt | Co | |
| 28 | Nickel | Ni | |
| 29 | Copper | Cu | |
| 30 | Zinc | Zn | |
| 31 | Gallium | Ga | |
| 32 | Germanium | Ge | |
| 33 | Arsenic | As | |
| 34 | Selenium | Se | |
| 35 | Bromine | Br | |
| 36 | Krypton | Kr | |
| 37 | Rubidium | Rb | |
| 38 | Strontium | Sr | |
| 39 | Yttrium | Y | |
| 40 | Zirconium | Zr | |
| 41 | Niobium | Nb | |
| 42 | Molybdenum | Mo | |
| 43 | Technetium | Tc | |
| 44 | Ruthenium | Ru | |
| 45 | Rhodium | Rh | |
| 46 | Palladium | Pd | |
| 47 | Silver | Ag | |
| 48 | Cadmium | Cd | |
| 49 | Indium | In | |
| 50 | Tin | Sn | |
| 51 | Antimony | Sb | |
| 52 | Tellurium | Te | |
| 53 | Iodine | I | |
| 54 | Xenon | Xe | |
| 55 | Cesium | Cs | |
| 56 | Barium | Ba | |
| 57 | Lanthanum | La | |
| 58 | Cerium | Ce | |
| 59 | Praseodymium | Pr | |
| 60 | Neodymium | Nd | |
| 61 | Promethium | Pm | |
| 62 | Samarium | Sm | |
| 63 | Europium | Eu | |
| 64 | Gadolinium | Gd | |
| 65 | Terbium | Tb | |
| 66 | Dysprosium | Dy | |
| 67 | Holmium | Ho | |
| 68 | Erbium | Er | |
| 69 | Thulium | Tm | |
| 70 | Ytterbium | Yb | |
| 71 | Lutetium | Lu | |
| 72 | Hafnium | Hf | |
| 73 | Tantalum | Ta | |
| 74 | Tungsten | W | |
| 75 | Rhenium | Re | |
| 76 | Osmium | Os | |
| 77 | Iridium | Ir | |
| 78 | Platinum | Pt | |
| 79 | Gold | Au | |
| 80 | Mercury | Hg | |
| 81 | Thallium | Tl | |
| 82 | Lead | Pb | |
| 83 | Bismuth | Bi | |
| 84 | Polonium | Po | |
| 85 | Astatine | At | |
| 86 | Radon | Rn | |
| 87 | Francium | Fr | |
| 88 | Radium | Ra | |
| 89 | Actinium | Ac | |
| 90 | Thorium | Th | |
| 91 | Protactinium | Pa | |
| 92 | Uranium | U | |
| 93 | Neptunium | Np | |
| 94 | Plutonium | Pu | |
| 95 | Americium | Am | |
| 96 | Curium | Cm | |
| 97 | Berkelium | Bk | |
| 98 | Californium | Cf | |
| 99 | Einsteinium | Es | |
| 100 | Fermium | Fm | |
| 101 | Mendelevium | Md | |
| 102 | Nobelium | No | |
| 103 | Lawrencium | Lr | |
| 104 | Rutherfordium | Rf | |
| 105 | Dubnium | Db | |
| 106 | Seaborgium | Sg | |
| 107 | Bohrium | Bh | |
| 108 | Hassium | Hs | |
| 109 | Meitnerium | Mt | |
| 110 | Darmstadtium | Ds | |
| 111 | Roentgenium | Rg | |
| 112 | Copernicium | Cn | |
| 113 | Nihonium | Nh | |
| 114 | Flerovium | Fl | |
| 115 | Moscovium | Mc | |
| 116 | Livermorium | Lv | |
| 117 | Tennessine | Ts | |
| 118 | Oganesson | Og |
Click here: for a schematic overview of the periodic table of elements in chart form
Do you need to know the weight of some molecules? Try our Molecular Weight Calculator!
Please report any accidental mistake in the above statistics on chemical elements
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Atomic Number 9
Copyright © 1998-2021 Lenntech B.V. All rights reserved
