The electrons of an atom are typically divided into two categories: valence and core electrons. Valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those occupying the innermost shell or lowest energy levels. This difference greatly influences the role of the two types of electrons in a chemical reaction. Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot. While core electrons are not involved in bonding, they influence the chemical reactivity of an atom.
The electron configuration of a oxygen atom is
[ce{O}: ,1s^22s^22p^4 label{1}]
which may be shorted
In the Lewis structure of PO43- there are a total of 32 valence electrons. For the Lewis structure for PO4 3- you should take formal charges into account to find the best Lewis structure for the molecule. Remember, PO4 3- has a negative three charge on the molecule. For the Lewis structure you'll need to have a total charge for the molecule of 3. Valence electrons are those in the The Correct Answer is. Outermost shell. Reason Explained. Outermost shell is correct for valence electrons are those in the. Valence Electrons The electrons in the outermost shell are the valence electrons the electrons on an atom that can be gained or lost in a chemical reaction. The valence electrons (i.e., the 2 s 2 2 p 4 part) are valence electrons, which do participate in the making and breaking of bonds. Similarly, in calcium (Equation 1.9B.3), the electrons in the argon-like closed shell are the core electrons and the the two electrons in the 4s.
[ce{O}:, [He]2s^2 2p^4 label{2}]
where the ([He]) stands for the configuration of helium ((1s^2)). Similarly, the configuration of calcium with 20 electrons can be written
[ce{Ca}:, [Ar]4s^2 label{3}]
where the ([Ar]) stands for the configuration of argon ((1s^22s^22p^6 3s^2 3p^6)). Electronic configurations that are the same as noble gases are very stable since they have a full octet (except helium with a full 1s orbital).
The (1s) electrons in oxygen do not participate in bonding (i.e., chemistry) and are called core electrons. The valence electrons (i.e., the (2s^22p^4) part) are valence electrons, which do participate in the making and breaking of bonds. Similarly, in calcium (Equation (ref{3})), the electrons in the argon-like closed shell are the core electrons and the the two electrons in the 4s orbital are valence electrons.

Example (PageIndex{1}): Cobalt
What are the core and valence electrons in cobalt?
Solution
Start by writing the electron configuration of cobalt with 27 electrons:
[1s^22s^22p^63s^23p^64s^23d^7 nonumber]
However, argon has the electronic structure (1s^22s^22p^23s^23p^6), so we can rewrite the configuration as
[[Ar]4s^23d^7 nonumber]
The two electrons in the (4s) orbital and the seven electrons in the (3d) are the valence electrons: all others are core electrons.
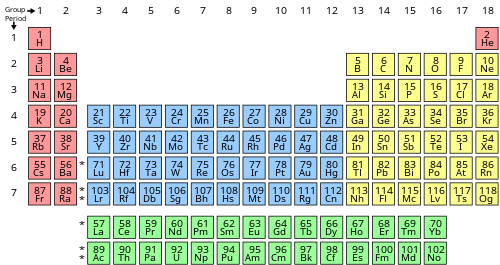
The periodicity of valance electrons can be seen in the Periodic Table. Basically, the periodicity is only applied to the main group elements, while in transition metals, rules are complex.
The core electrons remain the same in the increase of group numbers in the main group elements. On the other hand, the valance electrons increase by one from left to right of a main period, and remain the same down the column of a main group. This evolution gives periodical change in property of a period, and similar chemical property of a group, which is called periodical trend. The number of valence electrons in a main period is the same as its group number. The table below shows this rule clearly.
Under construction
Figure 1: 1A + 2A are metals. 3A to 8A are non-metals.
Calculate Number Density Valence Electrons
However, this periodicity cannot be applied to the transition group, which is more complicated than that of the main group. Although the outermost electrons can be easily determined, the apparent valence electrons considered in chemical reactivity are complex and fluctuated. Electrons going into d sublevel can play either a role of valence electrons or shielding electrons. So there is not always a certain number of apparent valence electrons. The number of apparent valence electrons for the first transition metal period is shown in the table below.
Under construction
Figure 2: Valence electrons for transition metals.
Relationship with Chemical Reactivity
The chemical reactivity of an atom is mainly determined by valence electrons. Atoms which have a complete shell of valence electrons tend to be chemically inert. Atoms with one or two valence electrons are highly reactive. This phenomenon can be explained by Hund's rule, which states that orbitals that are empty, half-full, or full are more stable than those that are not. For example, Ne is chemically inert because it has two valence electrons that fill its outermost shell which makes it stable compared to atoms such as Al, which has three valence electrons, but its valence electrons does not fill its outermost shell.
Although core electrons do not take part in chemical bonding, they play a role in determining the chemical reactivity of an atom. This influence is generally due to the effect it has on valence electrons. The effect can be observed from the gradual change of chemical reactivity in a group. As you go down a group, more shells are occupied by electrons, which increases the size of the atom. The more core electron shells an atom has, the larger the size of the atom, and the farther the valence electrons are from the nucleus, thus the valence electrons will experience less effective nuclear charge and will be easily lost. For example, (ce{Na}) and (ce{K}) can both react with water, but K has a more radical reaction because it has more shells of core electrons which makes the valence electron in its outermost orbital much easier to lose than the valence electron of Na.
References
- Miessler, Gary L., and Donald A. Tarr. Inorganic Chemistry. Upper Saddle River, NJ: Pearson Prentice Hall, 2010. Print.
- Brown, Ian David. The Chemical Bond in Inorganic Chemistry the Bond Valence Model. Oxford: Oxford UP, 2006. Print.
A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, a valence electron can only be in the outermost electron shell.
An atom with a closed shell of valence electrons (corresponding to an electron configuration (s^2p^6)) tends to be chemically inert. An atom with one or two valence electrons more than a closed shell is highly reactive, because the extra valence electrons are easily removed to form a positive ion. An atom with one or two valence electrons fewer than a closed shell is also highly reactive, because of a tendency either to gain the missing valence electrons (thereby forming a negative ion), or to share valence electrons (thereby forming a covalent bond).
Like an electron in an inner shell, a valence electron has the ability to absorb or release energy in the form of a photon. An energy gain can trigger an electron to move (jump) to an outer shell; this is known as atomic excitation. Or the electron can even break free from its associated atom's valence shell; this is ionization to form a positive ion. When an electron loses energy (thereby causing a photon to be emitted), then it can move to an inner shell which is not fully occupied.
The number of valence electrons
The number of valence electrons of an element can be determined by the periodic table group (vertical column) in which the element is categorized. With the exception of groups 3–12 (the transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular column.
| Periodic table group | Valence Electrons |
|---|---|
| Group 1 (I) (alkali metals) | 1 |
| Group 2 (II) (alkaline earth metals) | 2 |
| Groups 3-12 (transition metals) | 2* (The 4s shell is complete and cannot hold any more electrons) |
| Group 13 (III) (boron group) | 3 |
| Group 14 (IV) (carbon group) | 4 |
| Group 15 (V) (pnictogens) | 5 |
| Group 16 (VI) (chalcogens) | 6 |
| Group 17 (VII) (halogens) | 7 |
| Group 18 (VIII or 0) (noble gases) | 8** |
* The general method for counting valence electrons is generally not useful for transition metals. Instead the modified d electron count method is used. ** Except for helium, which has only two valence electrons.
The Concept of Open Valence ('Valence')
The valence (or valency) of an element is a measure of its combining power with other atoms when it forms chemical compounds or molecules. The concept of valence was developed in the last half of the 19th century and was successful in explaining the molecular structure of many organic compounds. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including Lewis structures (1916), valence bond theory (1927), molecular orbitals (1928), valence shell electron pair repulsion theory (1958) and all the advanced methods of quantum chemistry.
The combining power or affinity of an atom of an element was determined by the number of hydrogen atoms that it combined with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of two; and in hydrogen chloride, chlorine has a valence of 1. Chlorine, as it has a valence of one, can be substituted for hydrogen, so phosphorus has a valence of 5 in phosphorus pentachloride, PCl5. Valence diagrams of a compound represent the connectivity of the elements, lines between two elements, sometimes called bonds, represented a saturated valency for each element.[1] Examples are:-

| Compound | H2 | CH4 | C3H8 | C2H2 | NH3 | NaCN | H2S | H2SO4 | Cl2O7 |
| Diagram | |||||||||
| Valencies | Hydrogen 1 | Carbon 4 Hydrogen 1 | Carbon 4 Hydrogen 1 | Carbon 4 Hydrogen 1 | Nitrogen 3 Hydrogen 1 | Sodium 1 Carbon 4 Nitrogen 3 | Sulfur 2 Hydrogen 1 | Sulfur 6 Oxygen 2 Hydrogen 1 | Chlorine 7 Oxygen 2 |
Valence only describes connectivity, it does not describe the geometry of molecular compounds, or what are now known to be ionic compounds or giant covalent structures. The line between atoms does not represent a pair of electrons as it does in Lewis diagrams.
Further Reading
Khan Academy
Valence Electrons Are Found Where
Cliffs Notes
